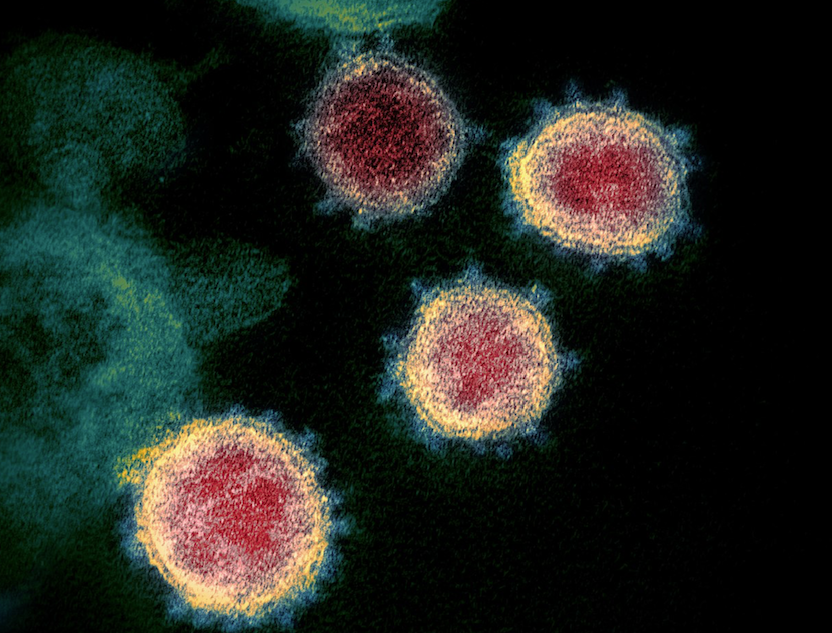
(Electron micrograph of SARS-CoV-2 from NIAID-RML)
It’s difficult to make predictions, especially about the future. That aphorism has been attributed to a long list of prognosticators, including the legendary wordsmith and NY Yankees catcher, Yogi Berra. Yogi was eminently quotable, including such gems as, “When you come to a fork in the road, take it.” But he wasn’t the first people’s scholar to have that insight into the difficulty of making predictions. It was apparently spoken in the Danish parliament, in 1937 or 1938, in Danish.
Today we are in a titanic, world-wide struggle against COVID-19. Nobody knows how it’s going to turn out — some have faith that existing technology will vanquish it; some see an existential threat to human life. Perhaps those are both wrong. Perhaps we will survive today’s battle and come to an uneasy stalemate with the disease. But then what? From existing knowledge, it’s likely that the virus responsible will not be eradicated easily.
The virus SARS-CoV-2, which causes COVID-19, appears to be profoundly resilient: it is highly infectious, somewhat mutable, but not so prone to mutation that it will suddenly decay. One theory about the 1918 ‘flu epidemic is that after the first wave passed, a second, even deadlier one resulted from a mutant variation of the virus. But this epidemic ended suddenly and without a clear reason; some have postulated that, because the virus responsible was so prone to mutation, the deadlier variant of the second wave, or a further mutant, lost its efficacy.
But I think there’s a good chance that humankind will mobilize its energy and intellect to devise means of driving the SARS-CoV-2 virus into semi-permanent submission. There are (at least) three kinds of approaches we might see to this end. One, or more, perhaps all, may do the job.
Let magic bullets fly
When the Human Immunodeficiency Virus was devastating the world and posing an existential threat to humankind, rescue came in the form of drugs. HIV is a retrovirus; it has an RNA genome that is copied into DNA and then inserts itself into the genome of the infected cell. The first useful drug interfered with “reverse transcriptase”, the enzyme that copies the RNA genome into DNA. Human cells don’t depend on a reverse transcriptase, so drugs that uniquely targeted this class of enzyme were effective and safe. Some of them were already known from basic research, and more were soon generated to help control the spread of AIDS.
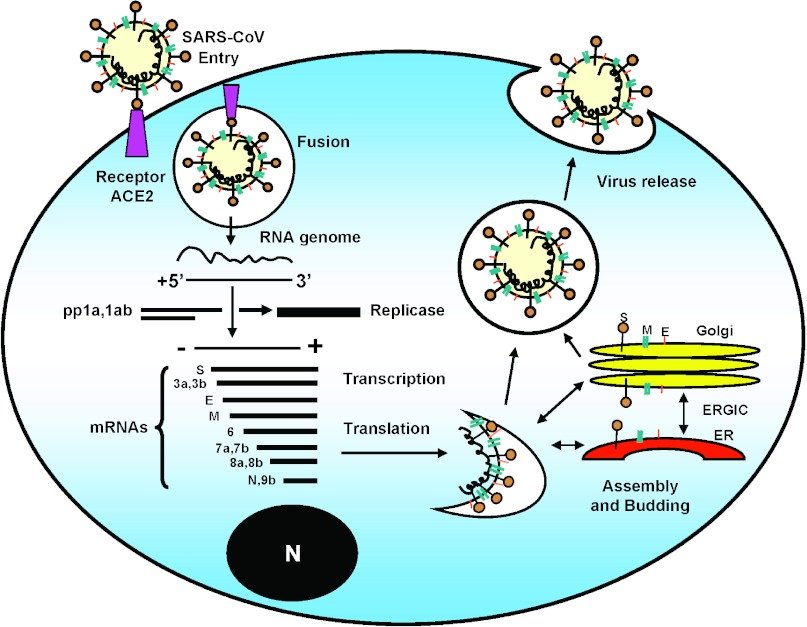
The use of reverse transcriptase inhibitors illustrates a basic principle of treating infectious diseases, diseases due to microbial agents like bacteria and viruses: targeting a part of their biology that’s different from that of their human hosts. Penicillin is a good example: it interferes with the need of growing bacteria to build a cell wall, a need that mammalian cells do not have. In the case of SARS-CoV-2, infection depends on an enzyme that copies its RNA genome into more RNA molecules (“RNA-dependent RNA polymerase”; “replicase” in the figure below), something mammalian cells also don’t need to do. Perhaps a drug can be found or created that will target this RNA-dependent RNA polymerase.
Viruses are mutable, and RNA viruses particularly so, for reasons I’ve explored elsewhere. HIV has a small genome, and it doesn’t have proofreading enzymes. So mutant variants that resisted the reverse transcriptase inhibitors soon appeared.
In a sequence that resembled MAD magazine’s classic “Spy vs Counterspy”, scientists soon identified new classes of inhibitors. Some of these worked by blocking the enzymes that cuts up the HIV proteins into the parts required for the working machinery of virus production (“protease inhibitors”). Others blocked entry of the virus into white blood cells; yet others inhibited the enzyme that integrates the DNA copy of the viral genome into the host cell DNA. The most effective treatment uses several of these classes of drugs at once (the “retroviral cocktail”), compounding their effectiveness. The idea is, that the virus may undergo a mutation to resist one kind of drug, but is unlikely to develop resistance to 3 classes of drugs at the same time.
Are there already magic bullets that target SARS-CoV-2? A number of drugs that were developed for other applications are currently being screened for effectiveness against the virus. Some of them have already passed Phase I clinical tests, indicating they are safe. There’s particular interest in Gilead Sciences’ drug remdesivir, which has shown antiviral activity against other RNA viruses, including coronaviruses. It is presently being used in small tests to see if it can stop COVID-19. Like the first class of HIV drugs, it is a nucleoside analogue.
A heavy infection of SARS-CoV-2 can lead to a “cytokine storm”, a massive reaction by cells of the immune system producing soluble inflammatory mediators. It is these mediators that cause damage, at least in some cases (1). Anti-inflammatory drugs may help control this burst of cytokines; indeed, some have already been used in limited circumstances.
If any of the currently available drugs, or new candidates, block infection in laboratory conditions, or limit subsequent inflammatory damage, they will be tested further. The idea that chloroquine, the antimalarial drug, might reduce inflammatory damage after SARS-CoV-2 infection is not without merit (even if Trump promotes it). It’s one of the drugs being tested.
The development of a new drug is a long and winding path, with failure possible at every step. We will probably need more candidate drugs, but the design and synthesis of these is well within the capability of industrial and academic research labs. Computer modeling of the enzymes essential for the viral life cycle, together with directed chemical and biological engineering, may give us a useful drug before a second wave of infections occurs.
Eternal vigilance
Until there are medicines to block the spread and pathology of coronavirus, monitoring of infections, social distancing, and contact tracing of infected people are the major tools for controlling the pandemic. These practices can be effective — they appear to be working in the Republic of Korea. That country, despite an early burst of cases, has subsequently maintained a very low number of daily cases and deaths (about 100 cases, fewer than 10 deaths).
Once the first wave of infections is brought down by current approaches, including isolation and frontline treatment, test kits must be produced in massive numbers, and used to identify COVID-19 infections. Those cases can be isolated, and contacts traced, to limit the scope of any outbreak. New screening kits are appearing, and the ability to detect and monitor infections is becoming faster and easier using the Polymerase Chain Reaction (2).
COVID-19 particularly harms victims who are immunocompromised, including those with “senescent immune systems”, i.e. the old. But it has also killed younger people in their prime, particularly those who have been exposed to high levels of the virus, including health care workers operating under semi-chaotic conditions. High levels of viral exposure, seeing one infected patient after another, may pose a particular problem. Immune systems are overwhelmed, even in a previously healthy person (3). Also, high viral loads are probably responsible for the “cytokine storm” already mentioned. As of today, more than 60 healthcare professionals have died from COVID-19 in Italy. If monitoring and tracing lead to smaller numbers of infections and fewer hospitalized patients, there should also be a decline in uncontrollable exposure for healthcare workers. Reducing the load by identifying outbreaks immediately and limiting their scope both reduces the level of disease overall, and also saves the lives of the helpers.
These two approaches, the development of effective drugs and containment by vigilance, will probably be the first approaches to controlling the disease.
Vaccination, the most efficient weapon of modern medicine
Vaccination profoundly increased the power of medicine. Many communicable diseases were essentially eliminated. COVID-19 vaccine development is undoubtedly going to be slower than drug development (although I could be wrong — predictions about the future are difficult), but it must be pursued. The American government recently announced that it is supporting two efforts by pharmaceutical companies to develop vaccines. Only once there is “herd immunity” to SARS-CoV-2 will we be relatively safe.
It’s sobering to remember that no effective antibody to HIV has been produced. This may be because the HIV is highly mutable (probably more so than SARS-CoV-2), and so presents a moving target. It is probably also due to the effectiveness of the antiretroviral drugs – immunization is not as critical.
A Phase I trial of an experimental vaccine has just begun in Seattle. The vaccine consists of small lipid particles containing an RNA that encodes a protein from the outer surface of the coronavirus. The protein chosen is part of the virus “spike”, which makes contact with its cellular receptor (see the figure above). This development will go on over the next year, and will establish whether the vaccine, at various doses, is safe in humans. However, this early study won’t be able to determine whether the vaccine is effective in blocking infection with SARS-CoV-2.
Vaccine development today has aspects that weren’t available decades ago. One of these is computer-based molecular modeling. Digital representation of viral antigens (the molecules that antibodies attach to and neutralize), allows for the computer-assisted design of antibody molecules that recognize them (4).
A potential site for blocking infection is the target of the virus, a cell surface protein called Angiotensin Converting Enzyme 2 (ACE2) (see figure above). We don’t yet know what effect an antibody against ACE2 would have on the body — ACE2 is part of a complex blood pressure-regulating network. Just yesterday I became aware of a study in which soluble ACE2 is used as a “decoy target” for the virus. Soluble ACE2 protein made by genetic engineering would soak up the virus, keeping it from attacking cells. This work is about to be published in the top journal CELL, and the results are encouraging: in cell culture, the technique reduces the production of virus 1,000 – 5,000 fold. This may be enough to give the body’s immune system a fighting chance (5). While not providing the continuing protection that active vaccination does, it may save lives by blocking further cycles of infection.
Development of a vaccine will take time. Dr. Anthony Fauci of the National Institutes of Health estimates that it will be at least 12-18 months before real efficacy trials can begin, even with the most favourable outcomes in the early going.
A relatively unsophisticated immunological approach is to identify and isolate antibodies in the blood of recovered COVID-19 patients. These may provide passive protection to vulnerable healthcare workers, or others. This was done successfully during the 1918 influenza outbreak.
Another approach was recently described: cells from recovered patients that make anti-SARS-CoV-2 antibodies can be transformed into eternal antibody producers by monoclonal antibody technology (6). If successful, this would provide such antibodies in quantities limited only by the volume of cell culture.
Many labs, around the world, are working on COVID-19. Surely human ingenuity will triumph? Of course, there are no guarantees. The plague arrived in Europe in the mid-fourteenth century, and was still killing Londoners three hundred years later. Why it then went away, nobody really knows. Let’s hope that we can do something to ensure that the history of SARS-CoV-2 is much shorter.
This Just In!
On June 18, 2021, the New York Times ran a story about a new development in the fight against COVID-19. The government of the USA is investing three billion dollars to find a pill that would stop an infection at the earlies stages (when there are just a few symptoms, such as loss of taste/smell) (the rationale for some of these drugs is described above).
Go to Latest Posts
Cited work
- “Why Some COVID-19 Cases Are Worse than Others“, K. Zimmer, TheScientist, Feb. 24, 2020.
- “How SARS-CoV-2 Tests Work and What’s Next in COVID-19 Diagnostics“, B. Nogrady, TheScientist, March 3, 2020.
- “How Does the Coronavirus Behave Inside a Patient?” Siddhartha Mukherjee, The New Yorker, March 26.
- “Computer-Aided Antibody Design: An Overview”, Y. S. Choong et al., Adv. Exp. Med. Biol. 1053:221 (2017).
- “Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2”, V. Monteil, H. Kwon, P. Prado, et al., CELL (in press)
- “Chinese scientists isolate ‘extremely effective’ antibodies that may help treat COVID-19” M. Q. Pollard, Globe and Mail, April 1, 2020.