Our bones, teeth and hair carry in them an encrypted GPS log of where we’ve been. If you lived in Seattle through your childhood and moved to Chicago ten years ago, that is indelibly written there. There will also be a record of what you’ve eaten recently. Advances in the science and technology of reading that information are having an impact on forensics, anthropology and agriculture, among other pursuits. At the heart of that technology lies a fundamental property of matter, which is that many of the atoms that make up all of creation come in subtle variations, variations that we are able to detect with great precision.
A cold case in Utah
In October, 2000, two duck hunters walking along the edge of the Great Salt Lake in Utah came upon a grisly discovery. In a shallow grave, they found a large plastic bag containing human female remains: 26 bones, a purple beaded necklace, an oversized T shirt, a skull and a long ponytail. Some remains had been scattered by animal predators. There were no clues to her identity, and for 12 years it remained a mystery for the Unified Police Department of Salt Lake City. But on August 7, 2012, the police announced that they had a positive identification. She was Nikole Bakoles, a 20-year-old woman who had lived in and around Salt Lake City. She was a mother, although because of troubles in her life she had lost custody of her daughter. At the time of the identification, her former boyfriend was in prison for property crimes, but after questioning him, the police indicated that he was not the focus of their investigation. Said Salt Lake County Sheriff Jim Winder, “Someone took this beautiful young girl and left her out in the mud in Salt Lake County. We want to find out who did it.”
The identification of the body of Nikole Bakoles took twelve years. For the first seven or eight years, the technology needed simply wasn’t available. Then in 2008, scientists at the University of Utah turned a powerful new scientific approach to the task, and a couple of years later, her identification was complete. The key technology was isotope ratio analysis, which depends on resolving the slight variations of common elements found in living organisms. Once the detectives had the strong lead to Bakoles’ identity that was provided by isotope ratio analysis, they compared the DNA found at the scene with that of her presumptive parents in Seattle, and settled the question of her identity.

What is isotope ratio technology, and how is it used for forensic analysis? It took a deep understanding of the nature of matter, and of the “isotope landscape” of the United States, and sophisticated engineering.
The heart of matter
Understanding the nature of matter, how it is built from the elements, and the structure of the elements themselves, marked the dawn of modern physics. Around the beginning of the twentieth century physicists abandoned the idea that matter was a homogeneous solid and adopted a model of the elements in which a central nucleus is surrounded by electrons and a huge amount of empty space. In that model, the nucleus contains two kinds of particles that give it its character; on the one hand are the positively charged protons, and on the other are uncharged particles called neutrons. Protons and neutrons have the same mass (the scientist’s preferred term for what non-scientists call “weight”), and often are present in the same number. The number of protons gives an element its identity; for example, carbon always has 6 protons, hydrogen just 1. The sum of protons and neutrons gives the element its “mass”; normal carbon has an atomic mass of 12 because it has 6 neutrons in addition to 6 protons.
As the instruments for exploring atomic structure became more powerful, the picture of matter became more complex. In particular, it became evident that many elements had more than one nuclear form, with the different forms characterised by different numbers of neutrons. That difference doesn’t change the chemical properties of an element: carbon is still carbon. But in some cases, the addition of one or more neutrons makes the nucleus unstable. Such a nucleus can undergo radioactive decay, emitting one or more radioactive particles or photons, and changing its atomic number and chemical nature thereby. Radioactivity was first seen with “heavy” elements, such as radium, where it is relatively easy to detect, in part because of its high energy. But in time even “light” elements such as carbon, hydrogen and oxygen, three of the most abundant atoms of living tissue, were found to have radioactive versions. A little later, variant forms of nuclei were discovered that had more neutrons than protons, but which were stable, not subject to radioactive decay. An example is carbon 13, symbolically 13C, which has 6 protons and 7 neutrons in its nucleus. Such stable isotopes are the basis for the analysis of the bones of Nikole Bakoles.
The three faces of hydrogen
The simplest of the elements, hydrogen, nicely illustrates the concept of isotope variation. The most common form of hydrogen contains just one proton in its nucleus, whose positive charge allows it to hold on to the single electron whizzing around it. Such a hydrogen nucleus has a mass of 1. There’s another kind of hydrogen in the world, called deuterium, which also has one proton in its nucleus, but it also has a neutron, so its mass is 2 (deuteros is “second” in Greek). Water containing deuterium is called “heavy water”, and is a modulator in some types of nuclear reactors. There are, on average, 6,420 atoms of hydrogen in the world for every atom of deuterium. Deuterium is a stable isotope of hydrogen. (There’s also a third form of hydrogen, the unstable, radioactive, tritium.)
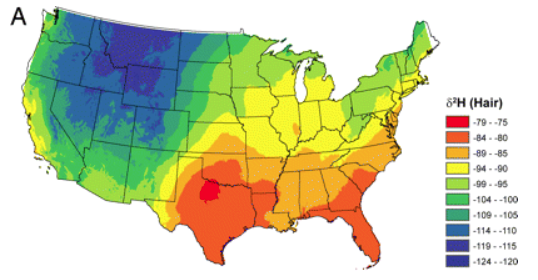
The key to using stable isotopes for identification is this: there is a variation in their ratio between geographic parts of the world, over land and over the oceans. The ratio of deuterium to normal hydrogen is different in Seattle, for example, and Salt Lake City. The reasons for this are complicated, and don’t matter for this story. The key is, that there are differences in the ratios of the common stable isotopes, and that those differences can be detected. That, and the fact that what we eat and drink reflects those isotope differences, and ends up in our tissues, including bones, teeth, and hair.
The ability to detect the ratios between the isotopic forms of elements, called Isotope Ratio Mass Spectrometry, is the essential technique that led to the identification of Nikole Bakoles. IRMS is briefly described in another part of this post.
Nikole’s story
When the Salt Lake City police turned to the scientists to try to identify the remains in their murder case, it was the long blond ponytail that provided the necessary material. Hair grows at a rate of about 1 centimeter per month, and it is a linear record of the isotope ratios present in the water of the places we’ve recently lived. In the case of the Jane Doe, her long ponytail was a record of years of residence. The hydrogen isotopes in her hair samples reflected that she had mostly lived in the Salt Lake City area in recent years, but had periodically spent time in the Pacific Northwest. The detectives therefore focused their efforts on these locales, in particular on missing persons reports from Washington state, and ignored leads that had come in from other parts of the country. They found that indeed there was a woman whose parents in Seattle had reported her missing years earlier. They had reported it to a police department outside Salt Lake City, where she had previously lived, unaware that she now lived in Salt Lake City itself. Once the sheriff’s office had that clue, it was simply a matter of comparing her DNA with that of her parents to establish her identify. The murdered woman was Nikole Bakoles.
Unfortunately, in terms of its solution, the murder case remains cold. Although much more is now known, there is still no strong suspect in the murder. Twelve years is a long time in a murder investigation. But at least her parents know her fate. The police continue to hunt and hope. (There’s an account of this story on the NOVA website here and an account in the Salt Lake City Tribune newspaper here)
You are what you eat
Isotope analysis is helping to solve another kind of mystery. At the time of the last ice age, about 14,000 years ago, wanderers crossed from present-day Asia to the Alaskan peninsula. The glaciers had stored up much of the world’s water, and ocean levels had fallen to expose a land bridge that allowed the first inhabitants of North America to cross over. As a result of low precipitation in the northern areas, there was, paradoxically, less glaciation there than further south, allowing the incomers to settle in the Alaskan interior. These people were the progenitors of all of the aboriginal groups of North, Central and South America.An
In 2006, a site was identified that had been inhabited around 11,500 years ago by a group of Paleoindians. Labelled the “Upward Sun River” site, it is located above the Tanana River flood plain in central Alaska. The Tanana empties into the Yukon, and some 1400 kilometers from the site, the Yukon empties into the ocean. In 2010, the cremated body of a child was discovered at the site. This discovery was international news, as reported in the New York Times.
Two other children’s remains were found at a deeper excavation level of the site, and these dated back to just after the end of the ice age, some 14,000 years before the present. There is evidence that the site was occupied, mostly in the summer, at least six different times over a period of hundreds or even thousands of years. The children in the later discovery were, in one case, 6-12 weeks old, in the other, just 30 weeks of gestational age — either a still birth or a premature newborn. Some remarkable evidence for the kinds of lives lived by those earliest inhabitants of North America has been uncovered, including tool use, food remains, household workings, and environmental conditions of the time.

A team led by Dr. Ben Potter of the University of Alaska in Fairbanks has been studying the Upward Sun River site, and one of their achievements has been to determine the composition of the diet of the inhabitants (Choy et al., Proceedings of the National Academy of Sciences, 113:9757 (2016), and Halffman et al., ibid. 112:12344 (2015)). Scrapings were removed from outdoor hearths, and several components were purified. These leftover materials from long-ago diets were then analyzed by isotope ratio mass spectrometry. Much of the information was provided by analyzing isotopes of carbon and nitrogen. These elements are useful because their isotope ratios in animals living on the nearby land, the fish native to the rivers, and the salmon that return to the rivers to spawn, are distinctive. (The reasons for isotope ratio differences are complex, involving cycling of materials between the atmosphere, rocks, rivers and the ocean itself.) The Potter team was thereby able to determine that the Upward Sun River people had been eating, among other foods, migrating salmon, and also how much of their diet this comprised. It also showed that salmon were already migrating 1400 kilometers upriver to spawn, almost as far north as they do today, even though glaciers had only recently receded from the area.
To complete the identification of the salmon used as food, analysis of the mitochondrial DNA found in the hearths showed that they were chum salmon. As predicted by the theory of human migration across the land bridge from Asia, the characteristics of the camp sites at the Tanana River are similar to those found on the Kamchatka peninsula in Russia, which also include buried human remains from the same time period. An exciting prospect for future work is the possibility of establishing links between these ancient peoples and present day populations by DNA analysis.
The Upward Sun River work illustrates the complementarity between stable isotope analysis and DNA sequencing. The isotope work is good for determining where a human or animal has been. DNA analysis can provide its genetic identification: who or what provided this sample, and who else was it related to? There is an informative frequently-asked-questions document about the Upward Sun River site and its inhabitants here.
What else is isotope ratio analysis good for?
A variety of elements other than carbon, hydrogen, oxygen and nitrogen are found in the human body, usually in small amounts. Maps such as the one in Figure 2 exist for each of these, and they are usually different for different elements. One of these elements is strontium, which sneaks into our bones because it looks a lot like calcium. Strontium is present in very small amounts in the soil, and it finds its way into our bodies through food, often as dairy products. (Atmospheric testing of nuclear weapons by the US and the Soviet Union in past decades added a radioactive isotope of strontium to the biosphere. This was ending up in children’s bones, posing a threat to their health. One of the most important achievements of the peace movement was to get those countries to stop atmospheric testing. A Nobel Peace Prizes was awarded for that.) Our earliest teeth, which erupt when we are babies, contain strontium embedded in the calcium phosphate mineral, and that strontium never leaves. The ratio of strontium isotopes in those teeth reflect where we lived for the first years of our lives. The strontium in our bones turns over as the calcium is exchanged, and reflects the past few months of our place of residence.
Isotope analysis has also been used to monitor food safety, wildlife poaching, the identity of explosives used in separate terror incidents, and drug trafficking routes. That last application is possible because the isotopes of common elements are different in the landscapes where poppies are grown in South-East and South-West Asia, and also Bolivia and Columbia.
Mass Spectrometry, the Coles Notes version
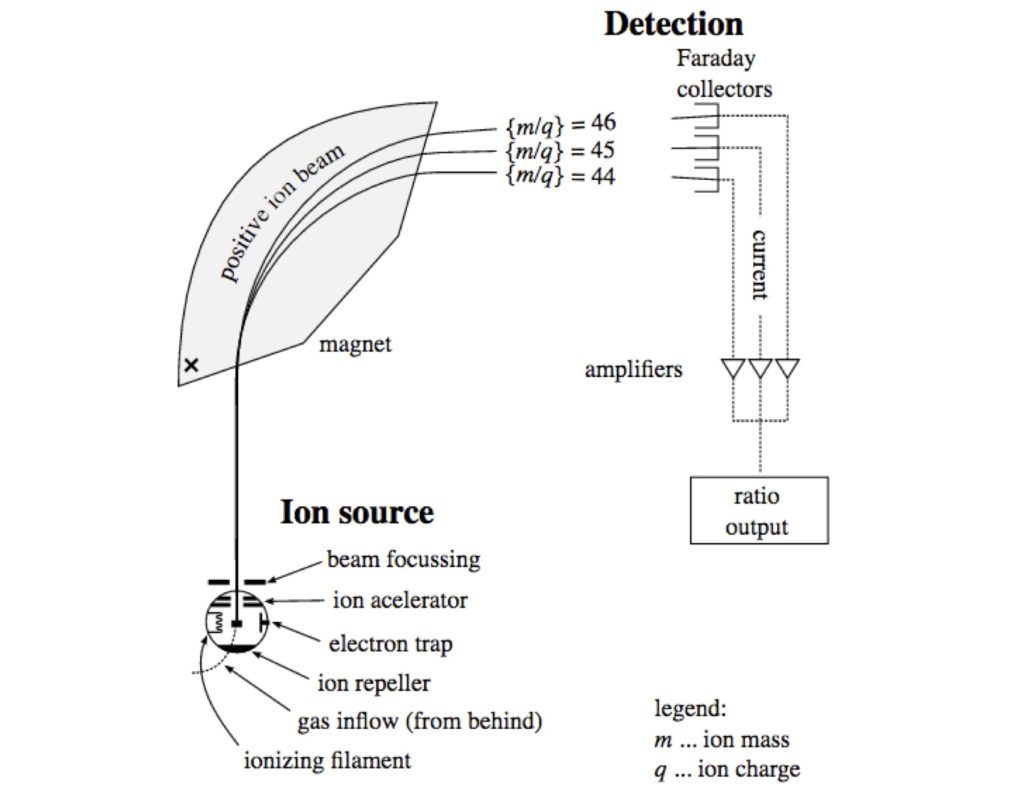
In the Isotope Ratio Mass Spectrometry (IRMS) technique used in the work described in this post, there are four components to the process. A sample of the material to be analyzed, which will usually have undergone purification beforehand, is combusted in an atmosphere containing oxygen. In the case of carbon isotope analysis, the resulting product is CO2. The sample is subjected to a spray of electrons which convert the molecules to positive ions, and the ionized products are accelerated by an electric field. Downstream, they enter a magnetic field perpendicular to their flight path, which causes the beam of ions to bend. The ions containing different isotopes of a given element have the same charge but a slightly different mass. Ions containing heavier isotopes (e.g. 13C) are deflected less by the magnetic field than lighter ones (12C). In the case of CO2 containing the common isotopes 12C and 16O, the mass is 44. If the carbon isotope is 13C, the mass will be 45, and so on. Finally, the beam of particles emerging from the magnetic field impinges on a detector.
IRMS machines are a lot more complex than the cartoon suggests, with components to remove unwanted materials from the ion beam before it is analyzed, and detectors that sense the exact location where the emerging ion beam impinges. Samples are constantly compared to standard materials having a known ratio of the isotope being analyzed. In the end, it is the absolute ratio of ions, such as the ratio of 13C to 12C, that is of interest.
Other types of mass spectrometric analysis are used for other purposes, often coupled to separation methods. But for the kinds of applications described here, it’s largely Isotope Ratio Mass Spectrometry that is used.
Go to Latest Posts